
Insights+: The US FDA New Drug Approvals in March 2022
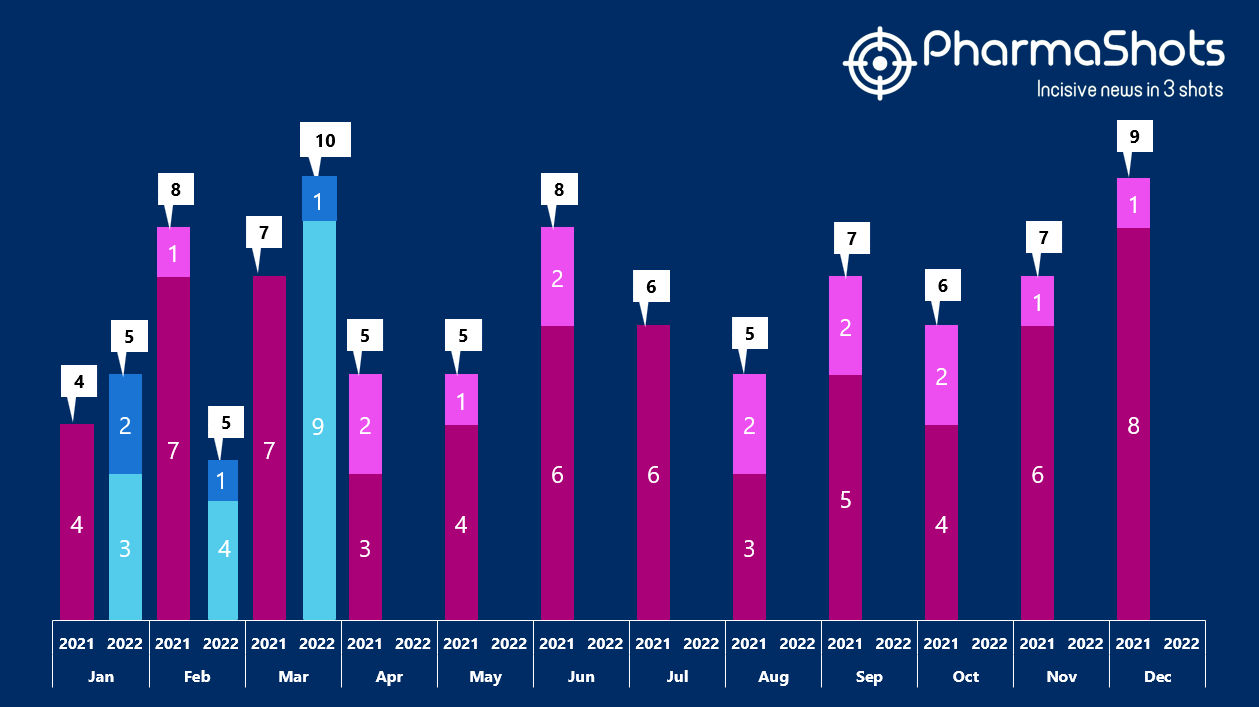
- The US FDA has approved 9 NDAs and 1 BLAs in Mar 2022, leading to treatments for patients and advances in the healthcare industry. The CDER and CBER approved 20 novel products in 2022
- In Mar 2022, the major highlights drugs were Adlarity for Alzheimer's Disease, Rinvoq for Ulcerative Colitis, Ztalmy for CDKL5 Deficiency Disorder
- PharmaShots has compiled a list of a total of 10 new drugs approved by the US FDA in March 2022
Adlarity
Active ingredient: donepezil hydrochloride Approved: March 14, 2022
Company: Corium Disease: Alzheimer's Disease
- The US FDA has approved Adlarity (5/10mg/day formulations, qw) for the treatment of AD. Additionally, patients may be switched from 5 or 10mg/day donepezil directly to Adlarity & the therapy is expected to be available in early 2022
- The new formulation of the therapy is delivered directly into a patient’s skin and bypasses the digestive system skin reducing the likelihood of adverse GI events associated with donepezil. The therapy has been developed using Corium's CORPLEX transdermal technology
- The therapy has the potential to substantially benefit patients, caregivers, and healthcare providers & provides effective, well-tolerated & stable dosing for 7 days who are unable to take oral donepezil
Rinvoq
Active ingredient: upadacitinib Approved: March 17, 2022
Company: AbbVie Disease: Ulcerative Colitis
- The approval is based on efficacy and safety data from P-III clinical studies (U-ACHIEVE and U-ACCOMPLISH) evaluating Rinvoq (45mg, qd, 8wks.) and 15/30mg, qd for the maintenance study (U-ACHIEVE maintenance) through 52wks.
- Rinvoq achieved 1EPs of clinical remission per modified Mayo Score [mMS]) @8 & 52wks, patients achieved 1EPs as early as week 2 and steroid-free clinical remission @1yrs.
- The study met all its 2EPs, including endoscopic improvement, HEMI, as well as corticosteroid-free clinical remission in the maintenance study. Rinvoq is a JAK inhibitor and this approval marks the first indication in gastroenterology
Tlando
Active ingredient: sutimlimab-jome Approved: March 17, 2022
Company: Antares Pharma Disease: Testosterone Replacement Therapy
- The US FDA has approved Tlando for TRT associated with an absence of endogenous testosterone or hypogonadism in adult males. The therapy is expected to be available in Q2’22 to provide a complementary treatment option to patients & clinicians
- The approval was based on a P-III study to evaluate Tlando (225mg, PO, BID for ~24 days) in 95 adult male patients which showed 80% achieved a 24hr. avg. serum testosterone concentration & had a maximum total testosterone concentration threshold ≤1620ng/dL b/w 1944/2700/2700ng/dL (82%/5%/0%), respectively.
- The company continues to support patient-centric care for different treatment options with an expanded commercial footprint
Perrigo’s Nasonex 24HR Allergy Receives the US FDA’s Approval for Nasal Allergy Spray
Nasonex 24HR Allergy
Active ingredient: mometasone furoate Approved: March 17, 2022
Company: Perrigo Disease: Nasal Allergy Spray
- The US FDA has approved Nasonex 24HR Allergy (mometasone furoate monohydrate) for over-the-counter use to prevent hay fever & allergy symptoms including allergic rhinitis, nasal congestion associated with seasonal allergic rhinitis, nasal polyps, and the prophylaxis of seasonal allergic rhinitis
- Nasonex (Rx-to-OTC switch) marks the first OTC formulation & is expected to be available at the end of 2022 with full prescription strength. Additionally, each nasal spray delivers 50mcg of mometasone furoate monohydrate delivering
- The company focuses on providing affordable self-care products to the patients with good quality strengthening internal innovation, R&D & regulatory capabilities
Ztalmy
Active ingredient: ganaxolone Approved: March 21, 2022
Company: Marinus Pharmaceuticals Disease: CDKL5 Deficiency Disorder
- The approval was based on the P-III (Marigold) trial evaluating Ztalmy vs PBO in 101 patients with CDD. The product is expected to be available in the US in July, following scheduling by the US DEA
- The trial meets its 1EPs i.e., reduction in 28-day major motor seizure frequency (30.7% vs 6.9%). In the (Marigold) OLE study, patients experienced a 49.6% reduction in major motor seizure frequency @12mos. & showed an efficacy, safety & tolerability
- The company is planning to launch ZTALMY One patient support program that enables the patients to access Ztalmy including assistance with product access, ongoing support to patients, caregivers & medical teams alongwith financial support
Opdualag
Active ingredient: nivolumab and relatlimab Approved: March 21, 2022
Company: BMS Disease: Melanoma
- The approval was based on the P-II/III (RELATIVITY-047) trial to evaluate relatlimab (160mg) + nivolumab (480mg) vs Opdivo (480mg, IV, q4w) alone in a ratio (1:1) in 714 patients aged ≥12yrs. with unresectable or metastatic melanoma
- The trial met its 1EPs i.e., improvement in PFS with m-PFS (10.1mos. vs 4.6mos.). The safety profile was similar to that previously reported for nivolumab with no new safety events, grade 3/4 drug-related AEs (18.9% vs 9.7%), treatment discontinuation due to AEs (14.6% vs 6.7%) & the combination resulted in increased T-cell activation
- Opdualag is the fixed-dose combination of nivolumab & relatlimab. The application has been approved under the US FDA’s RTOR pilot program
Pluvicto
Active ingredient: sutimlimab-jome Approved: March 23, 2022
Company: Novartis Disease: mCRPC
- The approval was based on a P-III (VISION) study that evaluates Pluvicto (7.4 GBq, IV infusion, q6w for a maximum of 6 cycles) + SOC vs SOC alone in a ratio (2:1) in 831 patients with PSMA+ mCRPC prior treated with AR pathway inhibition & taxane-based CT
- The results showed improvement in OS, 38% reduction in risk of death & reduction in risk of rPFS. Additionally, patients with evaluable disease at baseline demonstrated an overall response of 30% vs 2%
- Novartis anticipated the availability of Pluvicto to physicians and patients within weeks. The company is evaluating the therapy in two P-III studies in earlier lines of treatment for metastatic prostate cancer, intending to advance into earlier stages of the disease
Xelstrym
Active ingredient: dextroamphetamine Approved: March 23, 2022
Company: Noven Disease: ADHD
- The approval was based on a cross-over design, modified analog classroom study evaluating the safety and efficacy of Xelstrym vs PBO in pediatric patients aged 6 to 17yrs. with ADHD. The product is expected to be launched in the US as early as H2’22
- The primary efficacy EPs showed a significant separation from PBO with the use of Xelstrym while efficacy and safety were based on the comparable PK profile in adults and children and established a bridge to adequate & well-controlled studies of lisdexamfetamine.
- The product will be available in dosage strengths of 4.5mg/9hrs., 9mg/9hrs, and 13.5mg/9hrs. & 18mg/9hrs. The therapy also marks the 1st US FDA approved amphetamine transdermal patch for the same indication
Triumeq PD
Active ingredient: abacavir, dolutegravir, and lamivudine Approved: March 30, 2022
Company: ViiV Healthcare Disease: HIV
- The US FDA has approved NDA for Triumeq PD, a dispersible single-tablet regimen of the fixed-dose combination of abacavir, dolutegravir & lamivudine in children with HIV weighing 10kgs to <25 kgs
- Additionally, the US FDA has approved the sNDA to expand the use of Triumeq to lower the minimum weight of the children from 25kg to 40kg
- The MAA of Triumeq is currently under EMA’s review. Under the pediatric voluntary licenses, the company provides the generic versions of dolutegravir to manufacture and sold royalty-free for HIV patients in all least-developed, lower-middle-income, and sub-Saharan African countries along with upper-middle-income countries
Hyftor
Active ingredient: sirolimus Approved: April 4, 2022
Company: Nobelpharma Disease: Facial Angiofibroma in Patients with TSC
- The approval was based on a P-III study evaluating Hyftor 0.2% vs vehicle in 62 patients aged ≥6yrs. with facial angiofibroma associated with TSC for 12wks.
- The results demonstrated improvement from baseline in size & redness of facial angiofibroma. An assessment of improved or markedly improved showed a 50% & 75% reduction in size and a 2 & 3-level reduction in redness, 23% vs 6% were improved/markedly improved, 13% vs 35% were improved & 10% vs 3% were markedly improved
- Hyftor is supplied in 10g tubes containing 2mg of sirolimus/gram with expected availability in the US in the coming mos. The company plans to provide patient support programs to help eligible patients with the treatment
Related Post: Insights+: The US FDA New Drug Approvals in February 2022

This content piece was prepared by our former Senior Editor. She had expertise in life science research and was an avid reader. For any query reach out to us at connect@pharmashots.com














